
Pulpdent is a family owned and operated dental research and manufacturing company established in 1947 and committed to its founding principles of education, prevention and proactive dental care so that people can live healthier and more productive lives.
Pulpdent’s research and product development is directed toward unlocking nature’s healing powers with bioactive materials that mimic the physical and chemical properties of tooth structure, behave favorably in the moist oral environment, and maximize the potential for remineralization.


Founding Principles
Pulpdent’s founder, Dr. Harold Berk, practiced dentistry for almost 65 years and taught on the faculty of Tufts University School of Dental Medicine from 1946 to 2005. Dr. Berk’s core principles continue to guide the company:
Pulpdent products are manufactured in Watertown, Massachusetts, USA in compliance with the company’s Quality System and all regulatory requirements. Each year we renew our commitment to investing in research and new technologies, earning the trust of the profession, inspiring clinicians with new ideas and materials, and providing the leadership to accomplish these objectives.
Worldwide Distribution
Pulpdent’s expanding product portfolio is distributed globally through local distribution partners. Its growth has allowed it to expand the reach of its unique products that advance the standard of care.
Awards and Recognition
Nearly a Century of History
Pulpdent’s history is anchored in a commitment to original dental research. Over the years, this research has led to patented chemical technologies and SMART™ dental materials that actively improve oral health and change the industry paradigm for dental material standards.
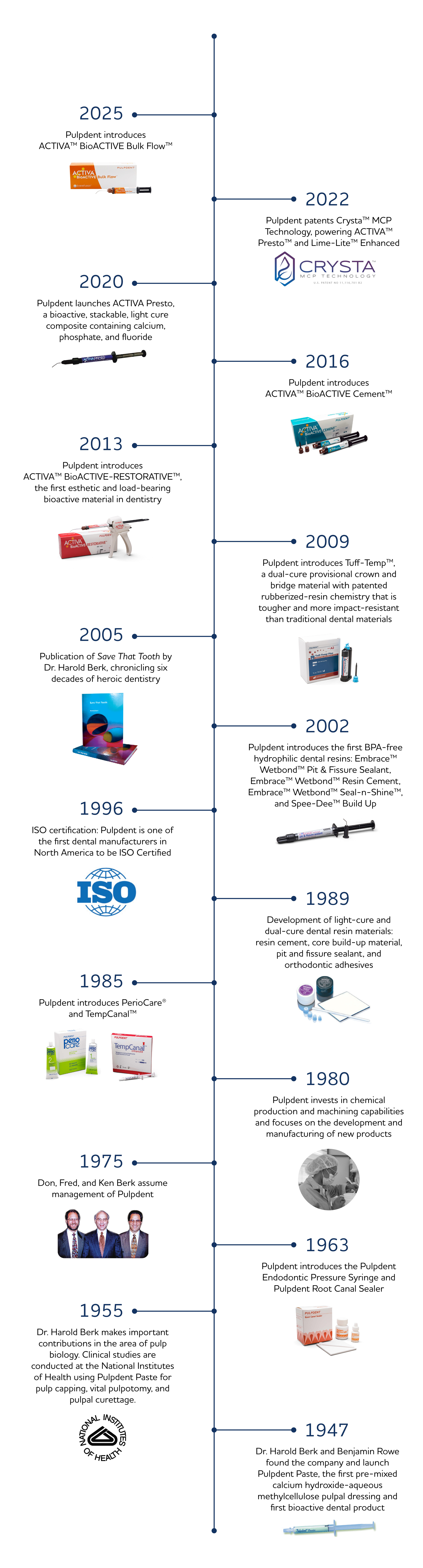
Pulpdent’s Timeline
Hover over each date to learn more
1947
Dr. Harold Berk and Benjamin Rowe found the company and launch Pulpdent Paste, the first pre-mixed calcium hydroxide-aqueous methylcellulose pulpal dressing and first bioactive dental product

1955
Dr. Harold Berk makes important contributions in the area of pulp biology. Clinical studies are conducted at the National Institutes of Health using Pulpdent Paste for pulp capping, vital pulpotomy, and pulpal curettage.

1963
Pulpdent introduces the Pulpdent Endodontic Pressure Syringe and Pulpdent Root Canal Sealer

1975
Don, Fred, and Ken Berk assume management of Pulpdent

1980
Pulpdent invests in chemical production and machining capabilities and focuses on the development and manufacturing of new products

1985
Pulpdent introduces PerioCare® and TempCanal™


1989
Development of light-cure and dual-cure dental resin materials: resin cement, core build-up material, pit and fissure sealant, and orthodontic adhesives

1996
ISO certification: Pulpdent is one of the first dental manufacturers in North America to be ISO Certified

2002
Pulpdent introduces the first BPA-free hydrophilic dental resins: Embrace™ Wetbond™ Pit & Fissure Sealant, Embrace™ Wetbond™ Resin Cement, Embrace™ Wetbond™ Seal-n-Shine™, and Spee-Dee™ Build Up

2005
Publication of Save That Tooth by Dr. Harold Berk, chronicling six decades of heroic dentistry

2009
Pulpdent introduces Tuff-Temp™, a dual-cure provisional crown and bridge material with patented rubberized-resin chemistry that is tougher and more impact-resistant than traditional dental materials

2013
Pulpdent introduces ACTIVA™ BioACTIVE-RESTORATIVE™, the first esthetic and load-bearing bioactive material in dentistry

2016
Pulpdent introduces ACTIVA™ BioACTIVE Cement™

2020
Pulpdent launches ACTIVA Presto, a bioactive, stackable, light cure composite containing calcium, phosphate, and fluoride

2022
Pulpdent introduces Pulpdent patents Crysta™ MCP Technology, powering ACTIVA™ Presto™ and Lime-Lite™ Enhanced

2025
Pulpdent introduces ACTIVA™ BioACTIVE Bulk Flow™
































Social Responsibility
Pulpdent has always had a special interest in public health and the oral health of children. We cooperate with the public health community and support many public health initiatives through Pulpdent’s Public Health Partnership Program. To request an in-kind donation for a public health initiative click here.